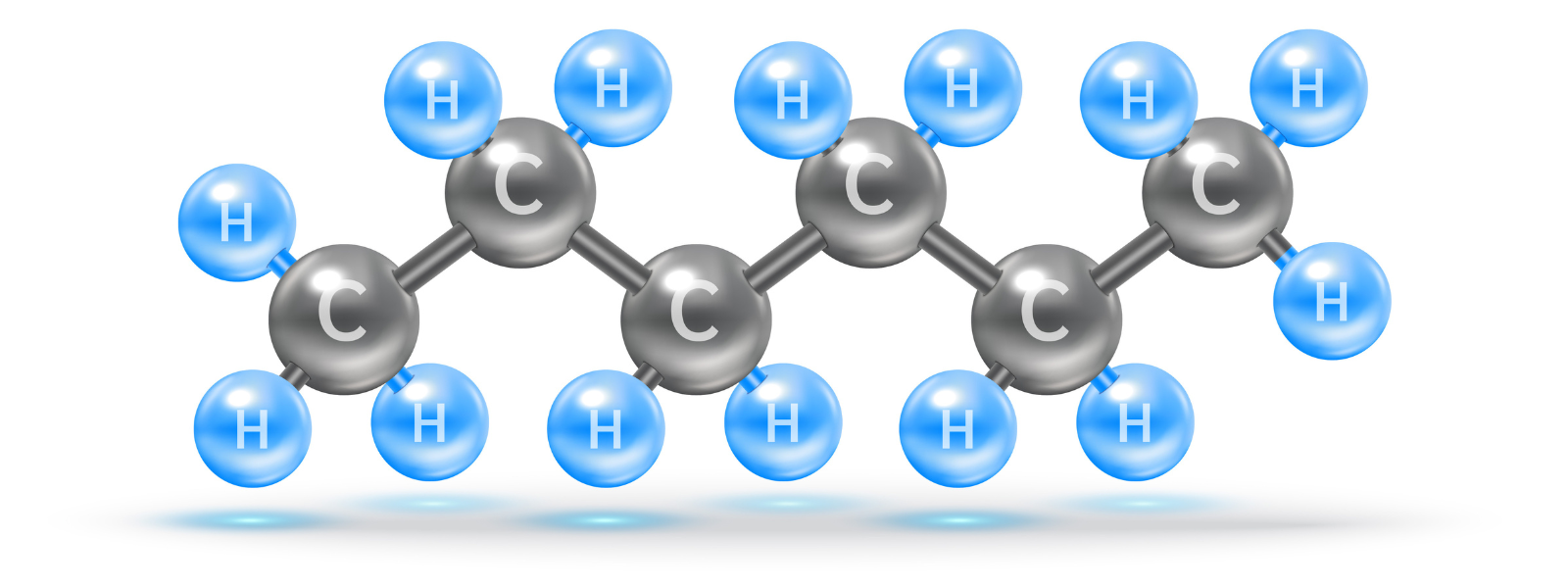
What is Hexane?
Hexane is a non-polar, clear liquid with a gasoline-like odor. But what does it mean to be non-polar? What is the chemical used for? Perhaps you just love chemicals, or you need to understand the meaning of polarity. Either way, keep reading for valuable information on both.
What Defines Polarity?
Polarity is all about how electrons are spread throughout a molecule. When one end of a molecule is more positive while the other is more negative, this is a polar molecule.
A molecule’s atomic structure can also determine if it is polar. If two similarly charged atoms are symmetrically aligned, their charges cancel out. Whether due to structure or atomic charge, non-polar molecules do not have much difference in electronegativity, so there is essentially no polarity present.
Hexane is non-polar due to being made of non-polar carbon-hydrogen bonds. While there is a slight difference in electronegativity between its hydrogen and carbon atoms, it is not significant enough to be polar. Even if there were polar bonds in the molecule, its symmetrical structure would cancel out the polarity.
Common Uses of Hexane
Hexane is a popular chemical in industrial settings and consumer products. It is used in a popular eco-friendly chemical oil extraction process for plant materials such as soybeans.
The substance is also used as a cleaning agent in the printing and textile industries and in industrial adhesives used for roofs and shoes. As for consumer products, hexane is most commonly found in paint thinner, rubber cement, and gasoline.
Want to order chemicals?
Take a look at our online inventory! No need to go back to your web search. Your order will be on its way after just one click. We sell bulk chemicals to keep your facility stocked and to cut down on packaging, making a move towards zero waste!
Have a question?
Let us know! Our representatives are knowledgeable and ready to assist. Rest assured that you’ll make the best decision with BulkChemicals2Go and reach out to us with your questions today!